FINALLY, AN attractive GMP TRAINING course !
Improve the GMP culture of the site -
Save money - Be serene during inspection
DO YOU DOUBT THE IMPACT OF YOUR CURRENT GMP TRAINING PROGRAM ?
3 SIMPLE STEPS TO IMPROVE THE SITE’S GMP CULTURE
Target messages for each employee profile
So that each employee
retains what is important to them
at THEIR workstation.
Give meaning to the message, the spirit of the law
So that the message is
simple, effective, and practical.
Train teams quickly with micro-learning
So that everyone can train at their own pace, in an agile way.
THE SPIRIT OF GMP
Training that has a profound impact on the quality culture of your site
WHAT PEOPLE ARE SAYING ABOUT SINFONY GMP TRAINING ?
We had the challenge of completing 3 GMP refresh training courses for our entire Belgian workforce. In 3 months, the work from the mastermind allowed us to be on time and as a bonus, we had a complete training course ready for our future recruits.
Thierry Dufrasne, Quality Director, Gosselies (Belgium)
Germain Nienhaus, Quality Assurance, Gosselies (Belgium)
There is no competition between labs on basic GMP training. We might as well take advantage of a training done by and for labs to use a modern video product, and free up time for more strategic training.
Daniel Greney, Quality Director, Kaysersberg (France)
Matthieu Ruas, Head of Quality Systems, Kaysersberg (France)
The basic GMP standard training is an opportunity for us to complete our program on quality culture. Thanks to micro-learning, we are now focusing on the key messages for our operators.
Eric Penn, Process and Knowledge Manager, Strasbourg (France)
Valérie Mathé, Quality Manager Strasbourg (France)
We had already done an experiment with Sinfony on the first 4 chapters. Now we have the entire training program, which we can configure as we wish in our LMS.
Françoise Bourniche, Qualified Person (pharmacist), Evreux (France)
Gaelle Canivet, pharmaceutical compliance expert, Evreux (France)
WATCH THE TESTIMONIAL SUMMARIES
THE POWER AND EFFICIENCY OF A CUSTOMIZABLE STANDARD COURSE
4 standard profiles (Leadership team members and N-1, first-line supervisors, field employees): reach all on-site employees with messages adapted to each level.
Each video for each chapter and for each profile is less than 5 minutes long.
To be the most effective for the student, each video is built with the same neuro-messaging methods as in web marketing.
Each module has a quiz, and an exercise to apply learning, adapted to each profile. Each exercise promotes discussion between hierarchical levels, to facilitate deployment of quality culture.
Watch the introductory video for the "field" level and the "first line supervisor"
1) Hosted standard version: we host the training for you, in a version of our LMS specially configured for you. You manage your employees’ assignments using the 4 standard profiles.
2) Hosted custom version: in addition to the hosted standard version, you can configure intermediate programs from the 4 standard profiles.
3) Version to embed in your LMS : you purchase the video source files (videos, scripts and Power Point). You can adapt the scripts and re-record the videos. You configure the modules yourself in your LMS.How to complete the program in our LMS ?
HOW MUCH DOES YOUR GMP TRAINING COST YOU?
The inefficiency of your current GMP training is certainly costing you far more than you think.
ATTRACTIVE TRAINING FOR A
STRONG GMP IMPACT ON THE GROUND


WHAT IS CONTAINED IN THIS ATTRACTIVE GMP TRAINING PROGRAM ?
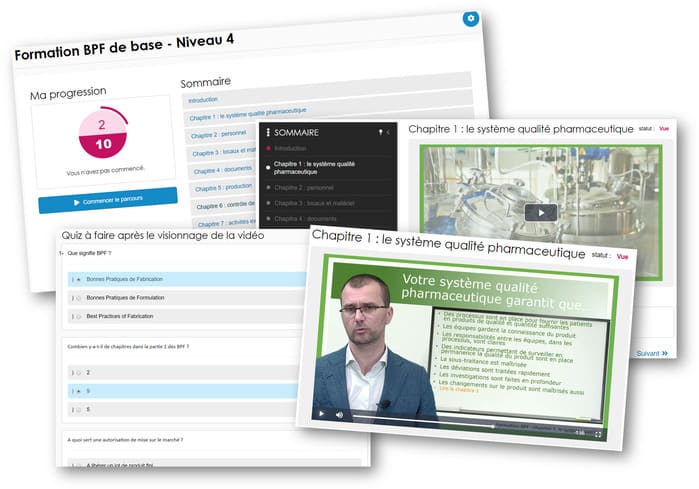
All of the 9 chapters are covered for the 4 profiles. There are a maximum of 9 videos for each profile, with a total duration of 25 minutes for operators or 45 minutes for directors. Less time than would be spent in any meeting. Some videos cover two profiles in the same chapter. The videos are between 2 and 7 minutes long, for quick and effective training. Each video is followed by a quiz adapted to the chapter and profile, and an exercise to be completed on the shop floor.
We help you to get started on the training with:
- Support for configuration and adaptation of the typical courses
- Help with content customization
- The possibility to re-record the videos in our studio
HOW TO USE THIS GMP TRAINING PROGRAM ?

Choose the type of hosting you want (your own or with us), and the desired personalization profile (standard or customized).
Assign a learner profile to each of your employees. Prepare communications announcing GMP training. Get the support of the Qualified person, the Quality Department and Site Management.
Directors, supervisors, field staff, newcomers... They can all follow their program at their own pace with complete autonomy.
Monitor the progress of training at the site level and intervene only if an employee is having difficulties.
Re-allocate resources saved to competitive or strategic activities.

"Let’s remove textual procedures that nobody reads and PowerPoint training that no one understands."...
Sinfony was created in May 2017, under the leadership of Olivier Depardieu, an international consultant with nearly 20 years of experience in the organization and reorganization of processes in the pharmaceutical, oil, and nuclear industries.
Noticing the disastrous consequences of inefficient procedures and training in large companies, Olivier sought inspiration in the unexpected world of solopreneurs of web marketing.
Olivier wanted to understand how young entrepreneurs in the digital world were able to sell training on the Internet. What were the mechanisms, based in neuroscience, that they were using?
From there, after understanding this world and far from the hushed atmosphere of industry corridors, Olivier launched exploratory tests in the banking and consulting industries to apply web marketing methods to internal training programs in large corporations.
In mid-2018, Sinfony entered the world of the pharmaceutical industry.
In mid-2019, Sinfony launched a mastermind with several pharmaceutical companies.
The goal? Establish a new GMP training standard that would be effective, modern, inexpensive, adaptable by any laboratory, and scalable to other parts of GMP than just part 1.
Challenge accepted!
CHEAPER THAN A COFFEE A MONTH, CHOOSE THE
"PLUG & PLAY" FORMULA THAT SUITS YOU
€8.900 (€1.5/month/user)
€10.900 (€0.9/month/user)
€13.900 (€0.58/month/user)
(Uniquely identified, one-shot license)
€15.000 (€30/user)
€20.000 (€20/user)
€30.000 (€15/user)
WHY IS YOUR NEW GMP TRAINING SO ATTRACTIVE?
Because thanks to neuro-messages, the spirit of GMP is understood and respected by all employees.
The messages are conceived using a method based on the way the brain works. These messages incorporate 4 key dimensions: why, what, how, and practice.
In addition, the messages are built around the personality of each profile, taking into account the context they are in, but also their fears, and motivations.
The messages therefore contain a strong emotional dimension.
However, the conception of messages is subtle, undetectable for people watching the videos. They naturally find the message pleasant and so retain it better.
Quizzes are a repetition of the content from the video, in another form. This also ensures good assimilation of the message.
The practical exercises are done to improve communication between hierarchical levels and improve the GMP culture on your site.
Refreshers are new content, established every year. For example, a video on the risk of contamination, on data integrity, or another hot topic from the year.
These refreshers are made during the annual GMP Day.
Refresher emails are pre-configured emails in the LMS, sent once a month to all users in your lab.
This email invites them to watch one of the videos which highlights the key points of the video's content. It is a kind of pre-recorded newsletter which helps to keep the GMP idea active in employees’ brains.
It is currently available in French, and English and will soon be available in Arabic, Spanish, and Italian.
This day is like a users club. It is used to collect and plan requests for new modules or languages and also to give feedback from users on the improvement of the modules.
Appendix 2 is available.
Appendix 1 will be available once the official version of the text is approved.
It depends on the option chosen. If you choose to connect with your business directory, then the access to GMP paths is automatic. If you chose the manual option, someone on your team will have administrator rights to carry out these updates.
Thanks to our LMS and our update process, your GMP training will always be up to date and always available.
Yes, we follow the updates of the official texts very closely. If we deem that these updates impact the content of the training, we will carry out the updates.
These updates are included in the annual recurring rate.
In addition, when a module is updated, the LMS keeps track of the version that users have viewed.
We would also like to discuss updates with our customers at the annual GMP Days.
Sinfony uses the Claroline Connect LMS, which is widely used in the institutional academic world.
This platform has existed for about twenty years, and is regularly undergoes technological updates.
The LMS falls into category 3 of the GAMP at most. The simplicity of the tool and the information managed, as well as the low to medium criticality level of the managed information, mean that a reduced validation is sufficient.
This means that when we install your version of the Sinfony GMP platform, you have a set of tests to run to check that it works properlyThe LMS that supports the GMP training is updated regularly by the owner, Claroline. Sinfony is entitled to accept or refuse the installation of an update. Our change control processes allow us to make controlled version upgrades of the LMS, with non-regression tests. All our customers will be informed in advance of the upcoming releases, and the measures taken by Sinfony to maintain continuity of service.
In accordance with the GDPR, the data we collect about your users belongs to you. Thanks to this data, you will know which user has viewed which information in which course. This allows us to prepare training progress reports for you, for you to consult. When you wish to terminate the contract with us, we undertake to return all your users' data to you, so that you can always show which user has followed which training module
Thanks to current technologies, you can distribute the content of the standard training in your LMS, without the user changing software. This question is a bit technical; our respective technical departments will discuss the best way to integrate the contents of our LMS into yours.
This feature means that for each chapter, you can embed additional videos or exercises that are unique to you. If, for example, you have had inspection remarks on water loops, then in chapter 3, you can add a video or an internal exercise to your laboratory, specific to you.
For the price of the cream in your coffee, you can use our LMS for all your other internal training.
Yes, the annual recurring price after the first year is used to pay for the completion of additional modules or languages. You will therefore be purchasing I a GMP training course that is not only always up to date but also is constantly being enhanced.
If you use the standard formula hosted by us, it is very fast, almost immediate. Just the time to load your user ID file into the LMS.
If you use the customized formula hosted by us, it takes time for our technical services and your IT department to connect the LMS to your directory which takes a few days.
If you purchase the licensed sources, you are solely responsible for the time frame you decide on.
Yes and no. No, because we want to prevent content from ending up, uncontrolled and for free on Youtube, which would be very detrimental to the image of the training and to the compliance of the laboratories. Yes, because you can buy them, they are then watermarked (an inscription of the license number is then engraved on each video).
Yes, you can order a content customization service including:
– Substantial changes to the background to adapt it to your culture.
– The PowerPoint layout to change to your company’s colors.
– A speaker of your company in the videos, coached by us.
However, this customization requires that all videos be re-recorded after editing the contents. Depending on the degree of customization you want, we will provide an estimate analysis of the cost of customization that you require in relation to the possible benefit.
We say "possible benefit" because today the laboratories participating in the mastermind have unanimously agreed that at their level, they do not need to personalize the contents. They do not see the benefit. So, it’s up to you to decide if the reward is worth the effort.
The price per month and per user makes it possible to give an idea of the cost that everyone can easily imagine: one coffee per month and per user. This is a ridiculously low price to ensure peace of mind for GMP training. Especially since the content will be enhanced over time, and that this enhancement is of course included in the price.
Pricing is calculated according to bands of a maximum number of users. At the billing level, the amount is invoiced annually at the beginning of the period according to the group of users. If during the year, the lab changes in size and changes pricing brackets, then let us know. In the event of growth, an invoice shall be issued on the date of the variation for the upper bracket, in proportion to the period of the year still to be covered. In the event of a decrease, a credit note is issued on the date of change for the lower bracket, and a refund is made.
